Key to Sample Handling and Zero-Contamination Workflow
Per- and polyfluoroalkyl substances (PFAS), widely known as “forever chemicals” that have been extensively used since the 1950s, have become a global focus of environmental and health regulation over the past two decades due to their widespread distribution in the environment and potential toxicity. As regulatory requirements demand detection at ultra-trace levels of ng/L (ppt), accurate detection and quantification (LC-MS/MS) pose the primary challenge for laboratories aiming to ensure water quality safety.
This article aims to discuss the regulation and testing of PFAS in water samples, including drinking water, surface water, and wastewater, and to explore how to optimize laboratory procedures and ensure regulatory compliance.
Table of Contents
What are PFAS?
Applications and Sources of PFAS
Health and Carcinogenic Risks of PFAS
Why the World is Starting to Focus on PFAS? The Emerging Environmental Challenge
PFAS Water Sample Testing Procedures
PFAS Testing Considerations: Contamination Prevention is Paramount
Frequently Asked Questions (FAQ)
What are PFAS?
PFAS (Per- and polyfluoroalkyl substances) are a large class of artificially synthesized fluorinated aliphatic compounds. Due to their high stability, they are widely used in consumer products and industrial fields. PFAS encompass over 10,000 compounds, with common and strictly regulated representative compounds including: Perfluorooctanoic acid (PFOA), Perfluorooctane sulfonic acid (PFOS), Perfluorononanoic acid (PFNA), and Perfluorohexane sulfonic acid (PFHxS).
PFAS possess extreme thermal and chemical stability, enabling them to resist water, lipids, high temperatures, light, and microbial degradation. Therefore, they have been widely applied in consumer products and industrial sectors since 1950. However, this extreme persistence makes PFAS difficult to decompose in the environment and prone to bioaccumulation in organisms. Hence, PFAS are referred to as “forever chemicals” and belong to the group of Persistent Organic Pollutants (POPs) regulated internationally.
Applications and Sources of PFAS
Due to their hydrophobic, oleophobic, and surface-active agent characteristics, PFAS are widely used in various products:
- Industrial Uses: Firefighting foams, semiconductor manufacturing processes, specialty chemical materials, etc.
- Consumer Products: Stain repellents for carpets, waterproof clothing, food packaging materials, non-stick cookware, cosmetics, paints, etc.
These PFAS-containing consumer products, manufacturing facilities, waste (such as landfills), and releases during use are potential sources of PFAS entering and polluting the environment (water, soil, sediment).
Health and Carcinogenic Risks of PFAS
The persistence of PFAS also leads to their widespread distribution in the environment and their potential entry into the food supply, subsequently appearing in humans and organisms. The primary source of human exposure is food ingestion. Excessive PFAS exposure may lead to a series of health problems and carcinogenic risks, including:
- Metabolism and Organs: Elevated cholesterol, increased liver enzymes, thyroid diseases, etc..
- Immune and Reproductive Systems: Immune system suppression, reduced fertility, neurological development issues and reproductive dysfunction in newborns, etc..
- Carcinogenic Risk: The International Agency for Research on Cancer (IARC) has classified PFOA as Group 1 “Carcinogenic to humans,” and PFOS as Group 2B “Possibly carcinogenic to humans.”
To reduce the harm posed by Persistent Organic Pollutants (POPs), the international community signed the Stockholm Convention starting in 2009, progressively listing PFAS and related compounds under regulation, with the aim of controlling and eliminating environmental contamination caused by POPs.
Why the World is Starting to Focus on PFAS? The Emerging Environmental Challenge
Although these PFAS substances have been widely used for decades, human understanding of the environmental behavior and toxicological effects of these compounds has deepened over the past twenty years.
Key characteristics exhibited by certain subgroups of PFAS make them a global regulatory focus:
- Potential for bioaccumulation: Found in the food chain and subsequently in the blood and tissues of humans and animals.
- High mobility in water, soil, and air: Leading to the widespread distribution of PFAS globally.
- Long-range transport potential: Traces of PFAS have also been found in remote polar regions.
- Eco- and toxicological effects on humans and the environment: Numerous studies have confirmed the impact of PFAS on the environment and health, and monitoring concentrations in many regions already exceed potential risk values.
- PFAS Drinking Water Regulations and Standards
Regarding PFAS, global regulation is becoming increasingly strict, with various countries setting relevant limits for PFAS in surface water and groundwater. For drinking water, regions such as the United States, the European Union, and Taiwan have either established or are in the process of establishing PFAS standards for drinking water and environmental water quality, as shown in the table below:
| Institution | Regulation/Standard | Maximum Contaminant Level (MCL) / Health Advisory or Guidance Value |
|---|---|---|
| Taiwan MOENV | Drinking Water Quality Standards | PFOA + PFOS ≤ 50 ng/L |
| PFOS + PFHxS ≤ 70 ng/L | ||
| US EPA | National Primary Drinking Water Regulations (NPDWR) | PFOA ≤ 4 ppt (ng/L) |
| PFOS ≤ 4 ppt | ||
| PFHxS ≤ 10 ppt | ||
| PFNA ≤ 10 ppt | ||
| HFPO-DA ≤ 10 ppt | ||
| Mixture of 2 or more*:Hazard Index ≤ 1 | ||
| European Environment Agency (EEA) | Drinking Water Directive (DWD) | Total PFAS ≤ 0.5 µg/L (500 ng/L) |
| Sum of 20 PFAS ≤ 0.1µg/L (100 ng/L) | ||
| World Health Organization (WHO) | WHO draft | PFOA ≤ 100 ppt (ng/L) |
| PFOS ≤ 100 ppt |
* US EPA Mixture Definition: For mixtures containing two or more of PFHxS, PFNA, HFPO-DA, and PFBS, the Hazard Index (HI) must be used.
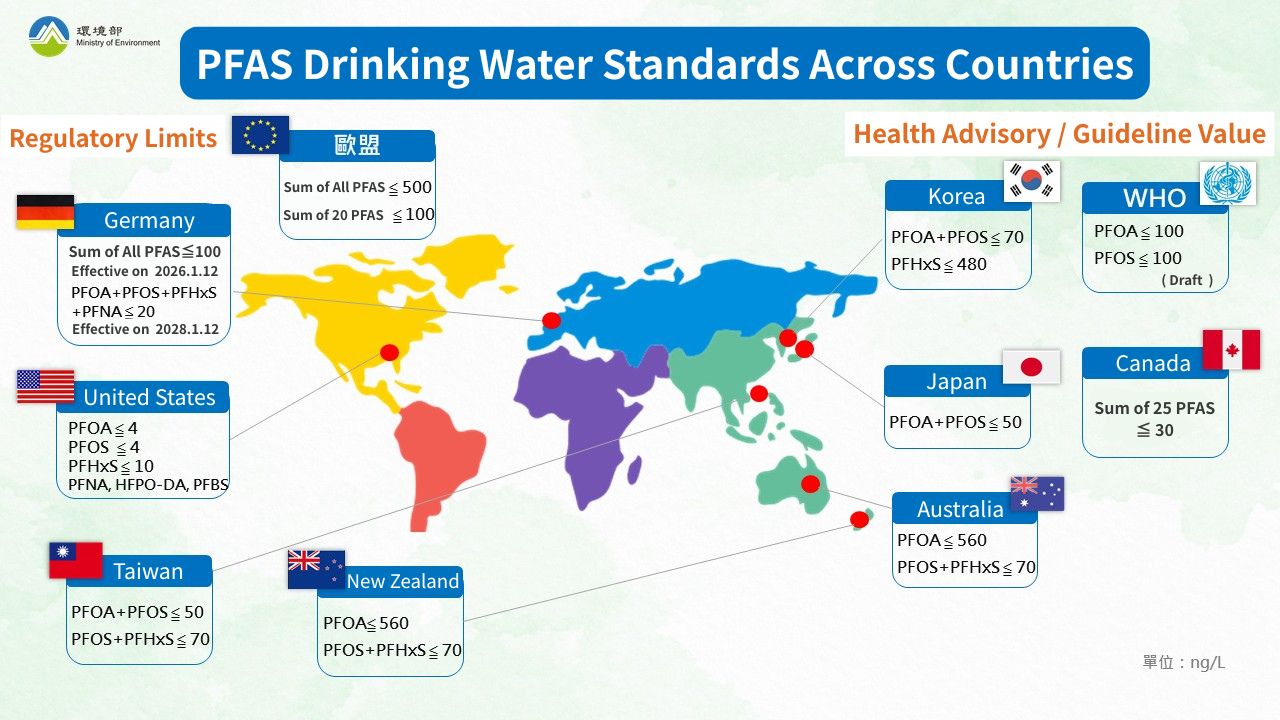
▲PFAS Drinking Water Standards Across Countries Image Source: Taiwan MOENV Water Quality Protection Web
PFAS Water Sample Testing Procedures
Due to the toxicity risk of PFAS and the need for ultra-low concentration detection (ppt level), testing methods are typically centered around Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). To achieve low Limits of Quantification (LOQ) and high accuracy, Solid Phase Extraction (SPE) is generally required first to concentrate the sample and remove matrix interference.
PFAS water sample analysis typically follows international methods such as US EPA Method 1633 or EPA Method 533/537.1. Taking the “Method for the Determination of Per- and Polyfluoroalkyl Substances in Water – Liquid Chromatography Tandem Mass Spectrometry” (NIEA W542.52B) as an example, PFAS water sample testing is generally divided into 4 major steps:

1. Sample Collection and Preparation
Polypropylene (PP) or polyethylene (PE) bottles should be used for sample collection, and glass bottles should be avoided to prevent PFAS adsorption onto the glass surface. NIEA W542.52B indicates that a surrogate internal standard solution should be added before sample pre-treatment.
2. Extraction and Concentration
• Direct Injection: For clean matrices, direct injection can be used, or combined with centrifugation and filtration for processing.
• Solid Phase Extraction (SPE): For complex matrices or when the target compound concentration is too low, SPE can be used to concentrate PFAS from large-volume water samples to achieve a low detection limit. The eluate is finally concentrated to dry or near-dryness using a nitrogen evaporator, then reconstituted in methanol and quantified.
* SPE Cartridges: Recommended use of Waters Oasis WAX 150 mg, Waters Oasis WAX Plus 225 mg, or equivalent.
Further Reading: Solid Phase Extraction (SPE))
3. Sample Clean-up – Selectively Performed
If interfering substances are present in the sample, appropriate pre-treatment procedures must be used to remove the interference, such as a clean-up step. For example, US EPA Method 1633 adds Supelclean™ ENVI-Carb™ bulk adsorbent after extraction for dispersive clean-up, followed by centrifugation and filtration to reduce the impact on LC-MS/MS analysis.
4. Analysis and Quantification
An appropriate PFAS analysis column should be selected to ensure separation efficiency and resolution, and analysis is performed using High Performance Liquid Chromatography coupled with Tandem Mass Spectrometry (LC-MS/MS). Quantification typically involves comparing the analyte with the internal standard to compensate for calibration losses and matrix effects, thereby improving accuracy.
* Chromatography Column: Recommended use of ACQUITY UPLC HSS T3 column, $1.8$ $\mu$m (particle size), $2.1$ mm (internal diameter) $\times$ $100$ mm (length), or equivalent.
PFAS Testing Considerations: Contamination Prevention is Paramount
The extreme persistence and ubiquitous nature of PFAS mean that laboratories must implement a zero-contamination workflow to avoid cross-contamination or background contamination, which is especially critical in trace analysis.
1. Containers and Equipment:
• Containers made of Polypropylene (PP) or High-Density Polyethylene (HDPE) should be used, rinsed with methanol and air-dried before use.
• Glass containers and pipettes should be avoided to prevent PFAS adsorption onto the glass surface, which can affect results.
• PP or PE vials should be used for on-instrument analysis.
2. Solvents and Water Quality:
• Only high-purity, PFAS-tested LC-MS-grade solvents must be used.
• Laboratory water must be ultrapure water (e.g., from a Milli-Q® water purification system) to ensure no detectable PFAS are present in the water.
3. Avoid Fluorinated Materials:
• Consumables and equipment containing fluoropolymers, such as PTFE and FEP, must be strictly avoided during the testing process.
4. Controlling Instrument System Contamination:
• Since the LC system’s pumps and tubing may themselves release PFAS background contamination, it is recommended to install a PFAS delay column when necessary to postpone the background PFAS from the instrument and reduce interference.
Reference Literature
- USEPA: Final PFAS National Primary Drinking Water Regulation
- EEA: PFAS pollution in European waters
- PFOS and PFOA in Drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality
- Water Quality Protection Web: PFAS You Need to Know, From Daily Life to Drinking Water
- Method 1633A, Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS
- Method for the Determination of Per- and Polyfluoroalkyl Substances in Water – Liquid Chromatography Tandem Mass Spectrometry” (NIEA W542.52B)
Frequently Asked Questions (FAQ)
Q1: Are Perfluoroalkyl substances (PFAS) present in the living environment?
A1: Investigation reports from multiple countries indicate the presence of PFAS in the environment. PFAS are widely used in waterproof, oil-repellent, and non-stick consumer products, food packaging, and specialty chemical fields. They are released into the living environment—including rivers, seas, sediment, soil, air, dust, and food—through related manufacturing processes, usage, and waste. Therefore, they are widely distributed in the environment.
Q2: Is PFAS analysis only applicable to drinking water?
A2: No. PFAS have been detected in various matrices, including: environmental water samples (groundwater, surface water, wastewater), solid matrices (soil, sludge, biological tissues), food, and beverages (bottled water, seafood, milk, livestock meat, and agricultural products), etc. However, this article only explains the PFAS testing procedure for water samples. Subsequent articles will delve into the detection of PFAS in food.
Q3: Can PFAS in water be removed by boiling?
A3: No, these chemicals cannot be removed by heating or boiling.
Q4: Can Pumps and Solid Phase Extraction Devices Cause Sample Contamination?
A4: Yes, international and domestic PFAS testing regulations clearly state the risk of contamination: “Contamination may originate from solvents, reagents, glassware, hardware used in sample processing, and LC system components,” and explicitly advise to avoid the use of fluoropolymer materials. To ensure testing accuracy down to the ppt level, contamination must be isolated at the source. Therefore, for Solid Phase Extraction (SPE), a PTFE-free SPE Vacuum Manifold should be used, along with a delay column to purify the background PFAS in the LC system, thereby establishing a reliable zero-contamination workflow.




