EVs Research Beginner’s Guide: 4 Steps from Sample to Results
Extracellular vesicles (EVs) have gained increasing attention in biology and medicine due to their great potential as biomarkers and therapeutic tools. However, the heterogeneity and complexity of EVs also bring many challenges to beginners.
Table of Contents
Why Read This First?
What Are Extracellular Vesicles and Exosomes?
EVs Research Workflow
Common Pitfalls for Beginners
FAQ
Why Read This First?
- Based on MISEV2023 guidelines, this article provides clear and practical guidance for newcomers.
- This serves as a “workflow navigation” to help you understand the fundamental steps of EV research.
- For in-depth technical details, refer to these articles:
• How to Purify Exosomes Using Tangential Flow Filtration (TFF)?• From Discovery to Validation: Why Do We Need “Exosome Standards”? (in Chinese)
What Are Extracellular Vesicles and Exosomes?
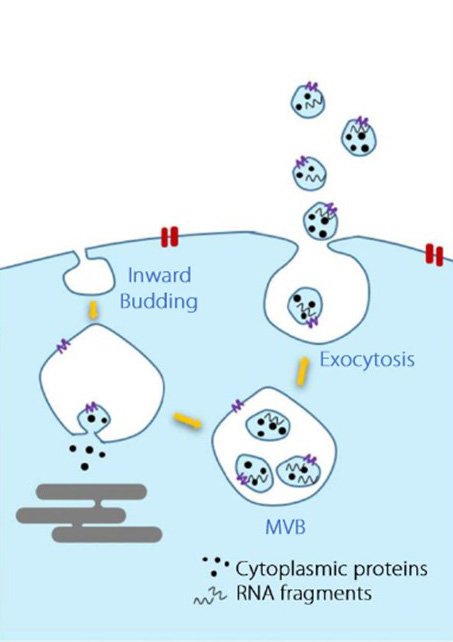
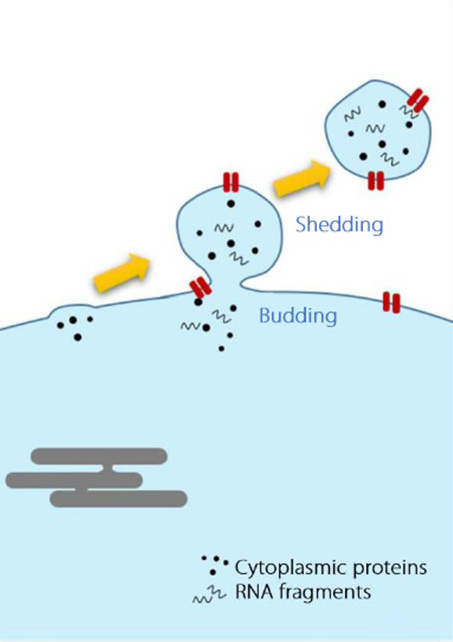
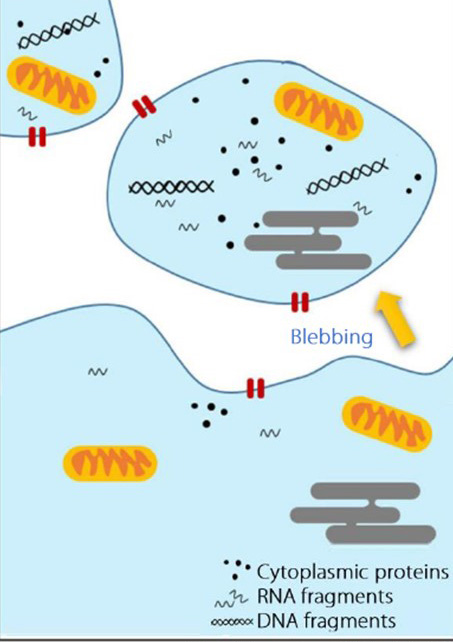
Extracellular vesicles (EVs) are particles released from cells, delimited by a lipid bilayer, and incapable of self-replication. Early studies classified EVs into three main types based on their biogenesis and size:
|
Exosomes |
Microvesicles |
Apoptotic Bodies |
|
|---|---|---|---|
 |
 |
 |
|
| Size | 40–120 nm | 100–1000 nm | 1000–5000 nm |
| Biogenesis | From multivesicular bodies (MVB), via endosomal pathway | Plasma membrane, via outward budding | Plasma membrane, during apoptosis |
| Markers & Composition | Tetraspanins (CD9, CD63, CD81), Alix, Tsg101, flotillin-1 | Selectins, Integrins | DNA, histones, organelles, nuclear fractions, Annexin V |
However, according to the International Society for Extracellular Vesicles (ISEV) in MISEV2018 and MISEV2023, if the biogenesis is unknown, it is recommended to use operational terms based on physical characteristics, surface markers, cellular origin, or morphology, such as sEV (small EVs), lEV (large EVs), or CD63+ EVs.
Exosomes refer specifically to EVs derived from the endosomal system and released via multivesicular bodies (MVBs). However, since it is often difficult to directly prove the biogenesis, the term should not be used without evidence. Their typical size ranges from 40–120 nm, and they are characterized by tetraspanins (CD9, CD63, CD81). Exosomes carry molecular information such as DNA, RNA, lipids, and proteins from parent cells, influencing recipient cells’ function and participating in many physiological and pathological processes.
EVs Research Workflow
EVs research involves several key steps—from sample collection to analysis and storage—all of which affect EV quality.
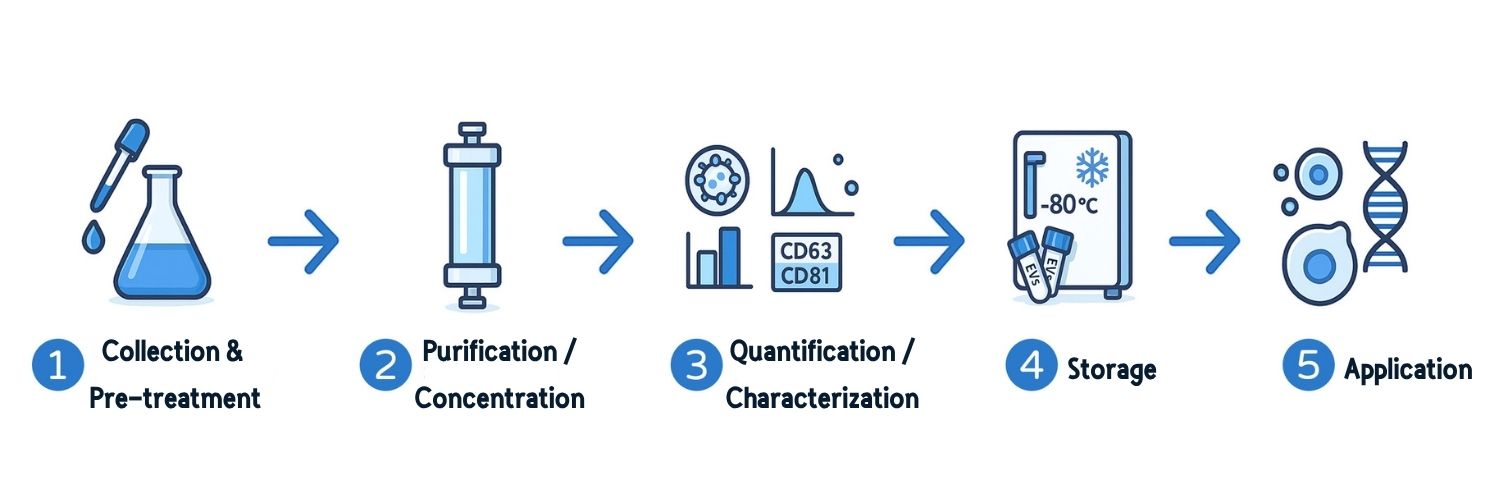
1. Collection and Pre-Processing
Purpose: Obtain biological materials containing EVs and remove cells or contaminants as early as possible.
Materials: EVs can be collected from cell-conditioned medium, bacteria, blood, urine, milk, and more.
General Recommendations:
• Report sample source, volume/weight, collection, and storage details.
• Remove cells early to avoid artifacts or compositional changes caused by damaged cells.
• Pre-process samples before storage to eliminate interfering substances.
• Avoid repeated freeze–thaw cycles.
• Document all storage conditions, preservatives, cryoprotectants, temperatures, durations, and thawing procedures.
* For sample-specific recommendations, refer to MISEV2023.
2. EV Separation and Concentration
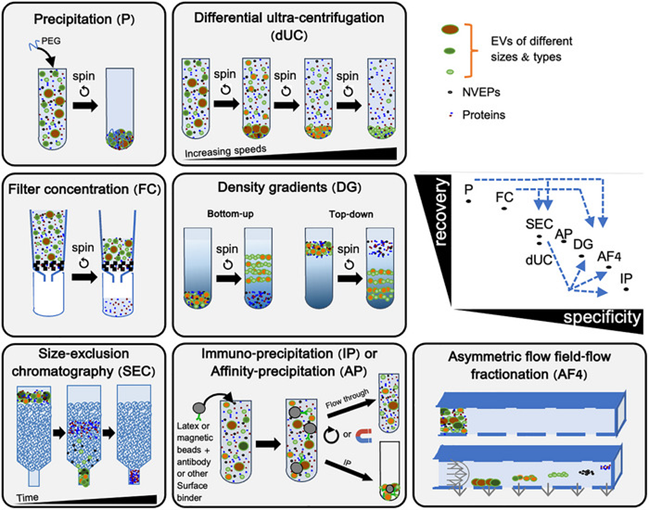
Purpose: Isolate and concentrate EVs from biological materials while minimizing non-EV contaminants such as free proteins or debris.
Methods: Common techniques include differential ultracentrifugation (dUC), density gradient (DG), ultrafiltration (UF), size exclusion chromatography (SEC), and precipitation methods.
General Recommendations:
• Choose methods according to sample type, research goal, and required purity/yield.
• Combine complementary techniques for higher purity.
• Pre-concentrate large volumes (e.g., urine, milk) before isolation.
• Prefer kits with transparent protocols.
• Report purity and recovery data.
• Provide full methodological details for reproducibility.
• Evaluate enrichment fold and yield after each step.
Further Reading: How to Purify Exosomes Using TFF?
3. EV Characterization
Purpose: Confirm that isolated particles are indeed EVs by analyzing size, morphology, and biomarkers.
Methods: Quantify particle concentration, protein/lipid/RNA content, and morphological features.
General Recommendations:
• Characterize EVs on three levels: (1) physical properties, (2) EV markers, and (3) non-EV contaminants.
• Use orthogonal methods with different detection limits.
• Report instruments, parameters, and data analysis methods.
• Define yield relative to biological source (cell count, volume, or tissue weight).
• Quantify particle count, protein/lipid content.
• Verify EV subtype-specific components when needed.
• Report LOD (limit of detection).
• Use standards, negative controls, and cross-platform validation.
Further Reading: Why Do We Need Exosome Standards? (in Chinese)
4. Storage
Purpose: Maintain EV integrity and stability for downstream use. Improper storage can alter EV properties, stability, and functionality.
General Recommendations:
• Report all storage details (preservatives, temperature, duration, thaw cycles).
• Avoid repeated freeze–thaw cycles; aliquot when possible.
• Rapid freezing and thawing are recommended to preserve EV morphology and function.
Common Pitfalls for Beginners
Due to their complexity, EVs research often traps beginners in common mistakes:
- Naming Confusion: Avoid using “exosome” without proving biogenesis; use “EVs” to prevent misleading conclusions.
- Sample Contamination: Serum or supplements contain exogenous EVs; use EV-depleted or serum-free medium.
- Cell Interference: Remove cells promptly after collection to avoid false EV-like particles.
- Improper Separation: Choose the right method based on sample type and application.
- Insufficient Characterization: Confirm with multiple methods; test for contaminants like lipoproteins.
- Improper Storage: Temperature and freeze–thaw damage EVs. Follow standardized protocols and record conditions.
References
- Minimal information for studies of extracellular vesicles (MISEV2023)
- MISEV2018 Guidelines
- Biological Functions Driven by mRNAs Carried by Extracellular Vesicles in Cancer
- Standardization of Sample Collection, Isolation and Analysis Methods in EV Research
FAQ
Q1: How should EVs be named when biogenesis is unclear?
A1: ISEV recommends using the general term “EVs” when subcellular origin is not confirmed, and naming based on physical characteristics, biochemical composition, or cellular origin to avoid confusion.
Q2: Which isolation method ensures the purest EVs?
A2: No single method yields completely pure EVs. Choose based on sample type and research purpose. Combining complementary purification techniques can improve both yield and purity.
Q3: How can I confirm that the isolated particles are truly EVs?
A3: Validate through multiple approaches: (1) physical properties (size/concentration), (2) EV-specific markers (transmembrane or GPI-anchored proteins), and (3) negative markers (non-EV co-isolated proteins) to assess purity.



