A Critical Factor in Pharmaceutical and Medical Device Safety
Bioburden, also known as microbial load or microbial count, refers to the total number of viable microorganisms (such as bacteria, fungi, or viruses) present on products, raw materials, or surfaces.It serves as an effective measure for assessing and controlling microbial levels in products, forming the foundation for quality assurance and playing a vital role in protecting patient and consumer safety.
Table of Contents
Why is Bioburden Testing Important?
What Are the Sources of Bioburden? How to Implement Bioburden Control?
Common Bioburden Testing Procedures and Methods
The Difference Between Bioburden Testing and Sterility Testing
Relevant Regulations: Domestic and International Standards
Why is Bioburden Testing Important?
Bioburden testing, also known as total viable count (TVC) or microbial limit testing, is a critical quality control (QC) process in the pharmaceutical and medical industries. It is used to detect and quantify the number of viable microorganisms present in a product prior to sterilization. This test reflects the quality of raw materials, the cleanliness of the production process, and the effectiveness of contamination control, while also serving as the basis for defining sterilization parameters and verifying sterilization efficacy.
Major regulatory authorities, such as the U.S. Food and Drug Administration (FDA) and the International Organization for Standardization (ISO), clearly mandate bioburden testing. For example:
- ISO 11737-1: Widely adopted as the bioburden testing standard for medical devices, recognized as a consensus standard by both the European Medical Device Regulation (EU MDR) and the U.S. FDA 510(k).
- United States Pharmacopeia (USP) <61>, <62>, <1115>: Cover microbial limits and control strategies for nonsterile products.
Bioburden testing is widely applied in high-risk industries such as medical devices, pharmaceuticals, biologics, as well as food and dietary supplements, serving as a critical step to ensure product safety and regulatory compliance.
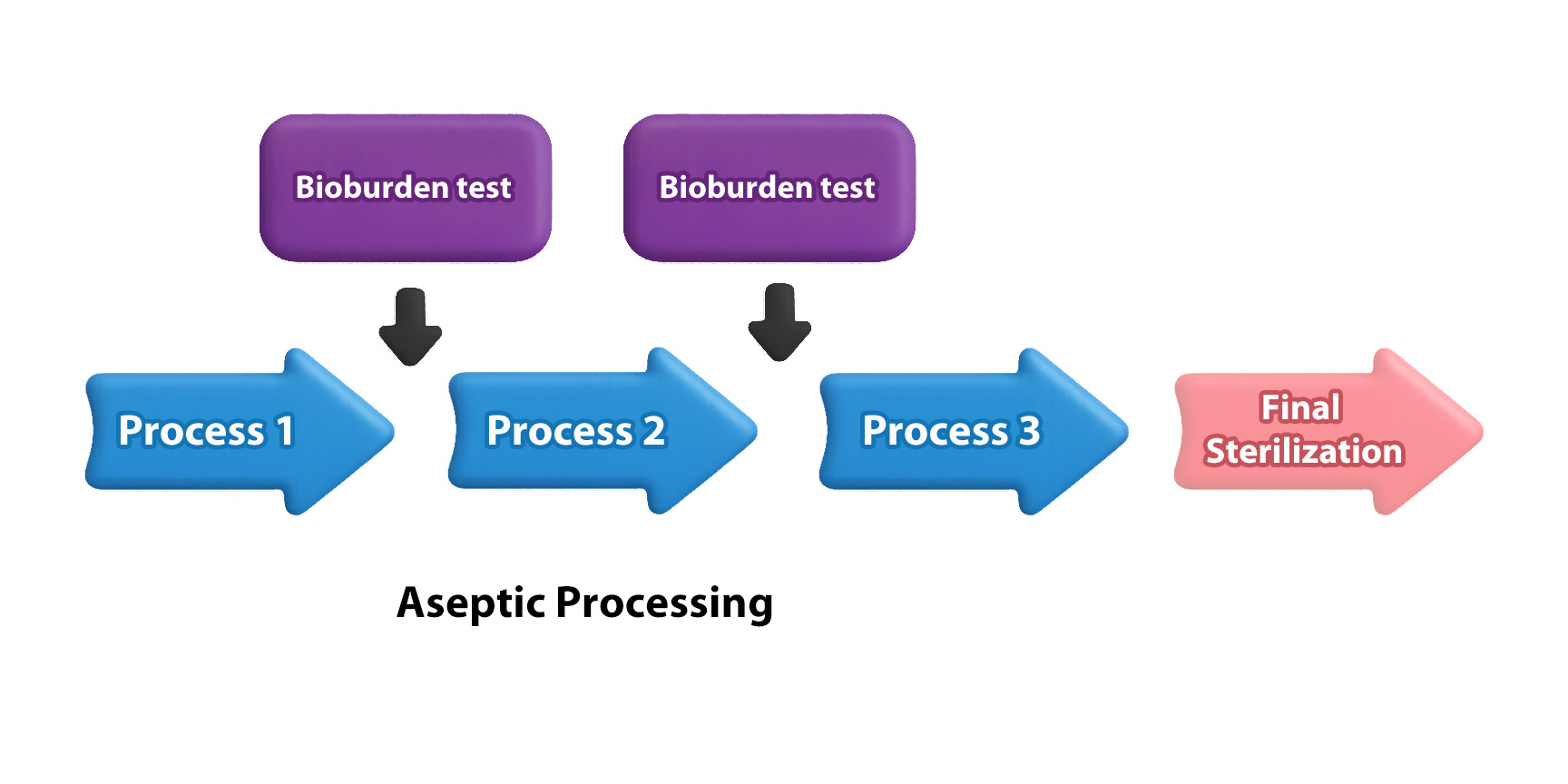
Bioburden testing is typically performed prior to terminal sterilization or before critical steps such as sterile filtration or filling within aseptic processing.The test results, indicating the microbial load, serve as an indicator of the overall cleanliness of the production process.Therefore, bioburden testing is regarded as a key step in process cleanliness validation and contamination control strategies, with the following critical implications:
- Sterilization Strategy Development:
The quantity and type of microorganisms directly affect the effectiveness of terminal sterilization. If the bioburden is too high, standard sterilization procedures may not completely eliminate all microorganisms. Therefore, the lower the bioburden level, the easier it is to achieve effective sterilization. - Quality Management and Control:
Regular monitoring of bioburden levels helps verify the stability of process cleanliness, identify weaknesses or contamination risks within the production process, and ensure the microbial content of the final product remains within regulatory limits. - Risk Assessment:
Bioburden testing assists in evaluating the potential infection risks that products may pose to patients or consumers. In particular, inadequate bioburden control in medical devices can lead to healthcare-associated infections (HAIs).Microbial characterization further helps detect the presence of endotoxins. Even after successful sterilization, some bacteria may release endotoxins, posing potential health risks to patients. - Regulatory Requirements:
Regulations in many countries and regions, such as ISO 11737-1, mandate that manufacturers of medical devices and pharmaceuticals conduct regular bioburden testing to comply with quality and safety standards.
What Are the Sources of Bioburden? How to Implement Bioburden Control?
Microorganisms can enter products or raw materials through various pathways.Strict monitoring of raw materials, personnel, equipment, and the entire manufacturing process is essential to maximize sterilization effectiveness and ensure the final product is safe for use.
Common sources of bioburden and corresponding control measures are summarized in the table below:
| Source | Control Measures |
| Raw Materials | Conduct microbial testing and control on raw materials |
| Manufacturing Environment | Maintain a clean production environment, such as cleanrooms and air filtration systems |
| Personnel | Wear sterile garments and follow strict hygiene protocols |
| Equipment and Tools | Regularly clean and disinfect equipment and work surfaces |
| Manufacturing Process | Follow aseptic operation standards and select appropriate sterilization methods for products |
Common Bioburden Testing Procedures and Methods
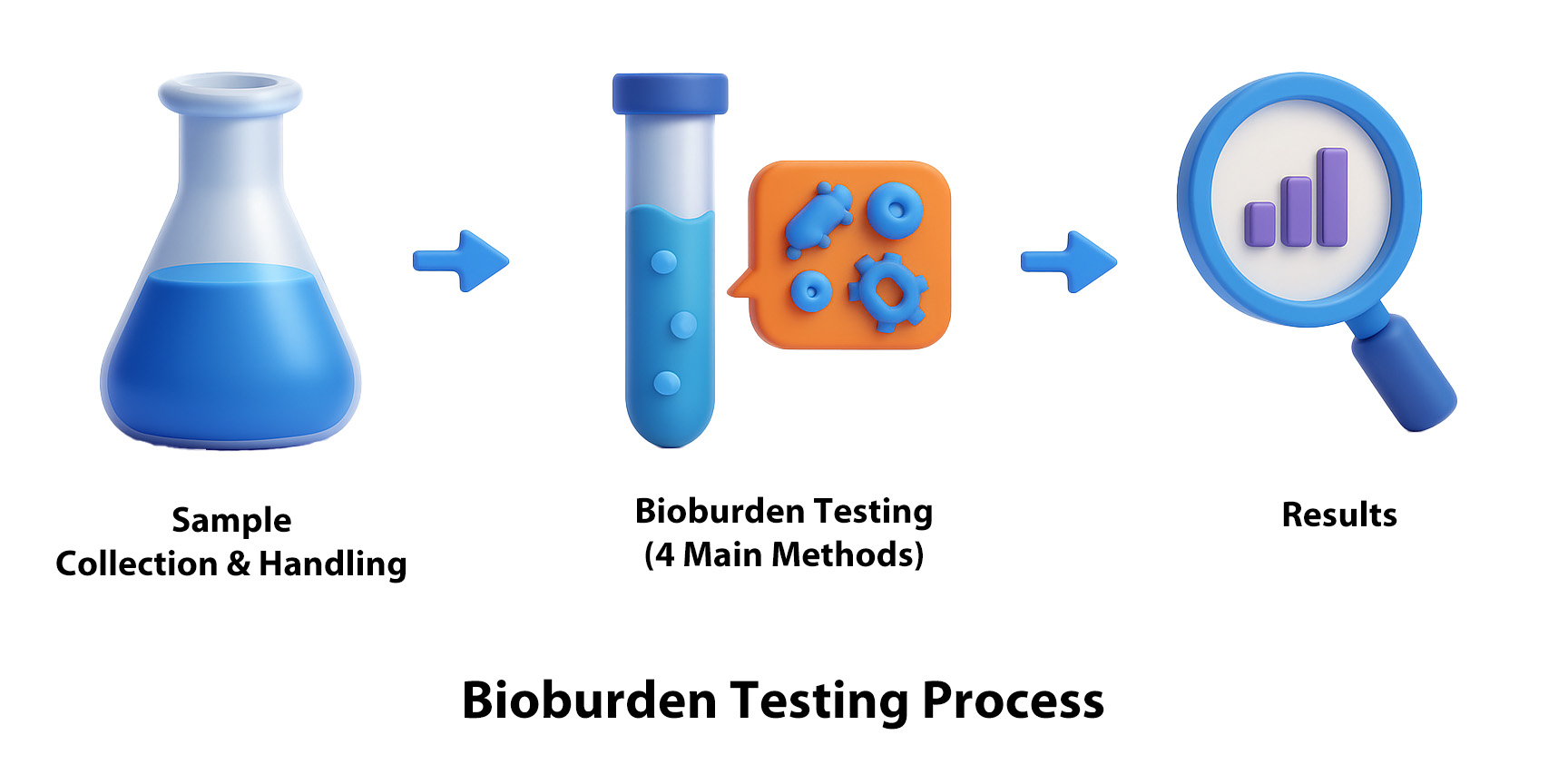
1. Sample Collection and Preparation:
Select an appropriate pre-treatment method based on the physical characteristics of the test sample to obtain the test solution.If no suitable method exists, an appropriate alternative procedure should be established.
| Sample | Preparation of Test Solution |
| Water-soluble Samples | Dissolve with an appropriate buffer or culture medium |
| Non-oily, Water-insoluble Samples | Dissolve with an appropriate buffer or culture medium; surfactants may be added |
| Oily Samples | Dissolve the sample in an appropriate ester solvent, then dilute with a preheated diluent |
| Spray-type Liquid or Solid Samples | Transfer the sample from the container to a membrane filtration device |
| Patch Samples | Place the adhesive side facing up in a sterile container, cover with sterile porous material, then transfer the patch into an appropriate diluent and shake for at least 30 minutes |
2. Selection of an Appropriate Testing Method:
To quantify bioburden, four mainstream quantitative analysis methods are commonly used: Membrane Filtration Method, Pour Plate Method (also known as the Dilution Method), Spread Plate Method, and the Most-Probable-Number (MPN) Method. The appropriate testing method should be selected based on the characteristics of the test solution.
| Membrane Filtration Method | Plate Count Methods | Most-Probable-Number (MPN) Method | ||
| Pour Plate Method | Spread Plate Method | |||
| Principle | The test solution is filtered through a 0.45 μm membrane, then the membrane is placed on culture medium for incubation | The test solution is mixed with non-solidified culture medium and poured into a petri dish for incubation | The test solution is spread evenly on the culture medium for incubation | The test solution undergoes at least three consecutive 10-fold serial dilutions, followed by incubation |
| Suitable Samples | Filterable large-volume liquid samples with low bioburden | Non-filterable samples, such as gels or viscous substances | Small-volume non-filterable samples | Products with extremely low bioburden |
| Suitable for Heat-sensitive Substances | Yes | No | Yes | Yes |
| Accuracy | High | High | High | Low |
| Incubation Time | Approximately 5 to 7 days | Approximately 3 to 7 days | Approximately 3 to 7 days | Approximately 3 to 7 days |
| Result Unit | Colony Forming Units (CFU) | Colony Forming Units (CFU) | Colony Forming Units (CFU) | Estimated based on MPN tables |
* The Most-Probable-Number (MPN) method is generally considered the least accurate among the available methods; therefore, it is only selected when no other method is applicable.
3. Result Interpretation:
Determine whether the product complies with regulatory standards based on the test results, or use the results to assess the required level of terminal sterilization.
Among these methods, membrane filtration is not only one of the most commonly used techniques for bioburden testing, but it can also help remove interfering substances from the sample.If the test sample contains substances that inhibit the growth or reproduction of microorganisms, such as antimicrobial agents or other components with antibacterial activity, it may result in false-negative results — meaning microorganisms are present but remain undetected due to growth inhibition.Therefore, when the characteristics of the test sample are suitable for membrane filtration, this method is often considered the preferred technique for bioburden testing.The procedure involves passing the test solution through a 0.45 μm membrane to capture microorganisms larger than the pore size, followed by transferring the membrane onto an appropriate culture medium to observe colony growth.
To accelerate the filtration process, ROCKER offers a variety of vacuum filtration systems compatible with 0.45 μm membranes. These systems can be equipped with stainless steel or plastic funnels and are specifically designed for bioburden testing.
The Ideal Choice for Bioburden Testing: Multi-Branch Filtration System — Enhancing Throughput and Efficiency
| MultiVac 301 – MB – A Vacuum Filtration System |
SolarVac 1201 – MB – T Vacuum Filtration System |
|
|
|
|
|
The Difference Between Bioburden Testing and Sterility Testing
Bioburden testing and sterility testing are two distinct yet related tests used in the medical device and pharmaceutical industries to ensure product sterility. They differ in purpose, timing, and testing methods.
| Bioburden Testing | Sterility Testing | |
| Purpose | Quantify and monitor the total viable microbial load during the manufacturing process | Confirm the sterility of the final product |
| Testing Timing | In-process checkpoints prior to final sterilization | After final sterilization of the product |
| Operating Environment | General laminar flow cabinet | Sterility testing isolator |
| Cleanliness Class 100 (Grade A) | ||
| Testing Methods | Membrane Filtration Method Pour Plate Method Spread Plate Method Most-Probable-Number (MPN) Method |
Membrane Filtration Method Direct Inoculation Method |
| Test Samples | Products before sterilization Non-sterile products |
Final products Products requiring sterility |
| Sample Types | Pharmaceuticals (solid and liquid forms) | Pharmaceuticals (solid and liquid forms) Medical devices |
| Test Results | CFU count (microbial load) | Sterile / Non-sterile |
| Application of Results | Evaluate sterilization dose and parameters; microbial monitoring, trend analysis, and process risk assessment | Verify product sterility; one of the bases for final product release |
| Standards | ISO 11737-1 USP <61> USP <62> |
ISO 11737-2 USP <71> |
In summary, bioburden testing is a preventive tool for process control, providing information to understand and manage the microbial load of a product prior to sterilization. In contrast, sterility testing is a validation and quality assurance tool aimed at confirming whether the final product meets sterility requirements after undergoing sterilization.
Further Reading: Sterility Testing
Relevant Regulations
Bioburden testing and result interpretation must follow international standards to ensure accuracy.
Common international standards include:
ISO 11737-1, United States Pharmacopeia (USP) <61>, <62>, <1229.3>, and Good Manufacturing Practice (GMP) guidelines.
It is worth noting that microbial limits vary depending on the product type, route of administration, and sterilization method.For non-sterile products, it is essential to demonstrate that the bioburden remains within acceptable limits to avoid potential risks to human health.
Applications
- Medical Device Industry: Surgical blades, catheters, sutures, artificial joints, etc.
- Pharmaceutical Industry: Sterile injectables, pharmaceutical raw materials, biologics (vaccines, antibodies, cell therapy products), etc.
- Food Industry: Environmental monitoring in food production facilities, water sample testing, etc.
- Cosmetics: Raw materials for cosmetic products.
References
- Fundamentals of Bioburden testing, MERCK
- ISO 11737-1: Sterilization of health care products – Microbiological methods – Part 1: Determination of a population of microorganisms on products
- Somethin’ Buggin’ Ya? Get the Basics of Bioburden Testing, Sartorius
- Regulatory Standards for Sterility Testing, Sartorius
- USP <61> MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: MICROBIAL ENUMERATION TESTS
- USP <62> MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS
- USP <1115> BIOBURDEN CONTROL OF NONSTERILE DRUG SUBSTANCES AND PRODUCTS
- USP <1229.3> Monitoring of Bioburden
FAQ
Q1: What is Bioburden Testing?
A1: Bioburden testing is a method used to estimate the number of viable microorganisms present in or on materials or surfaces. It is commonly applied in the pharmaceutical, medical device, and food industries as a critical quality control step prior to sterilization.
Q2: What is the difference between Bioburden Testing and Sterility Testing?
A2: Bioburden testing measures the total number of viable microorganisms present in a product before sterilization. Sterility testing, on the other hand, verifies whether the final product is sterile by determining if any microorganisms remain after sterilization.
Q3: Which testing method should I choose?
A3: Depending on the nature of the sample and the expected microbial load, you may select the Membrane Filtration Method, Plate Count Method, or the Most-Probable-Number (MPN) Method.
Q4: Is it mandatory to use a 0.45μm membrane for bioburden testing with membrane filtration?
A4: Not necessarily. USP <61> recommends using a 0.45μm pore size membrane for filtering liquid samples, as it effectively captures the majority of bacteria.Although the 0.45μm membrane is the most commonly referenced filter type in microbiological methods, a 0.2μm membrane may also be used for monitoring when smaller microorganisms are present in water samples.Additionally, in the food and beverage industry, larger pore size membranes such as 0.8μm or 1.2μm are sometimes used for microbial analysis, especially when detecting larger microorganisms like yeast and mold.
Q5: Which equipment should I use for bioburden testing?
A5: For high-volume sample testing, a Multi-Branch Filtration System (such as SolarVac 601 – MB – A) is recommended. For small-batch testing, a single-station stainless steel filter holder (such as WaterVac 101 – MB) can be used.
Q6: Does bioburden testing need to be performed under sterile conditions?
A6: Yes, bioburden testing should generally be performed under sterile or controlled environmental conditions to prevent background contamination from affecting the results.









